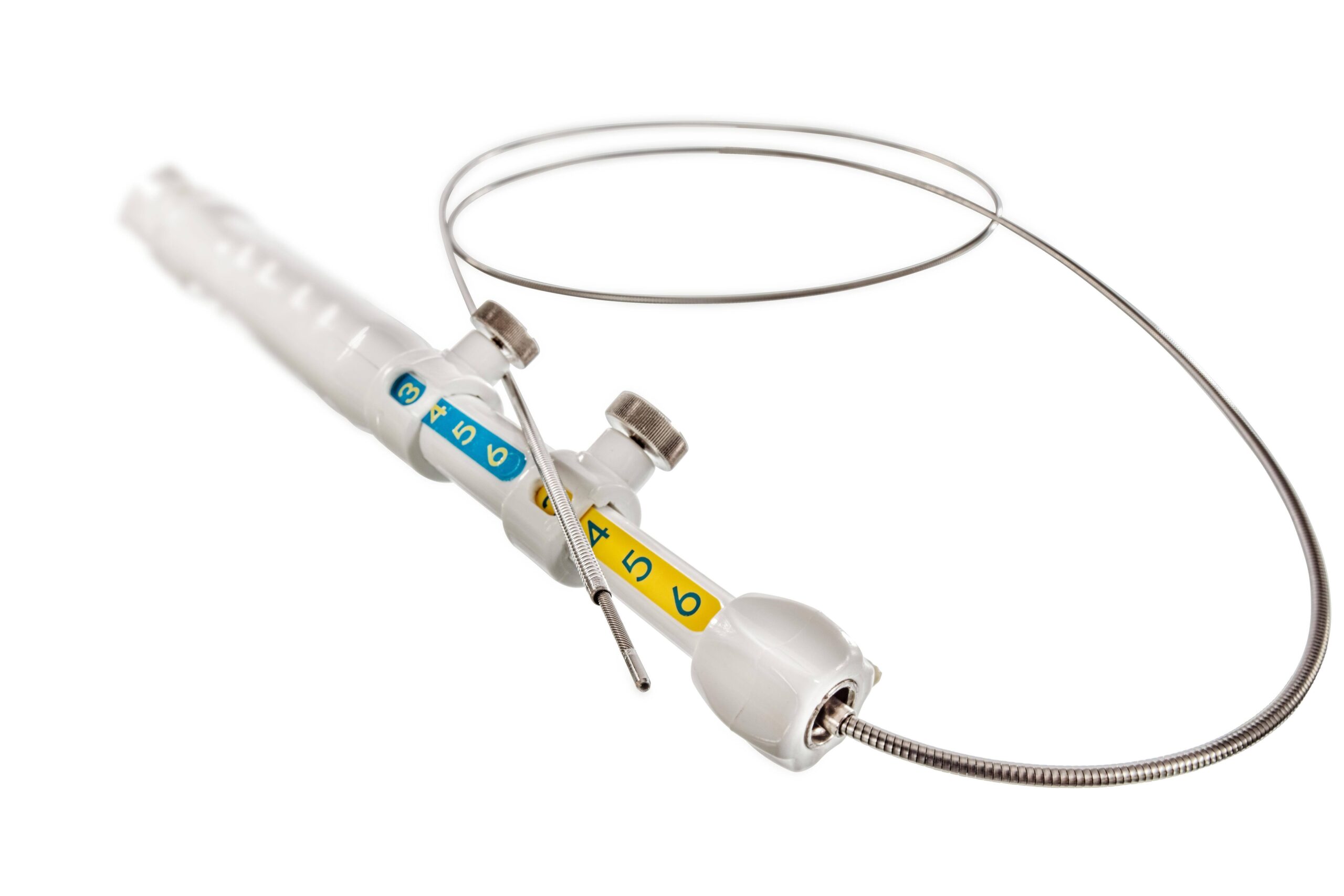
Powered rotation is the key to improved biopsies
Unlike conventional manual needle instruments, EndoDrill® features a motorized, rotating drill-tip cylinder. This design enables deep, precise tissue sampling. One or more solid core biopsies are extracted, providing the tissue architecture required for complete histological diagnosis, staging, and genetic analysis.